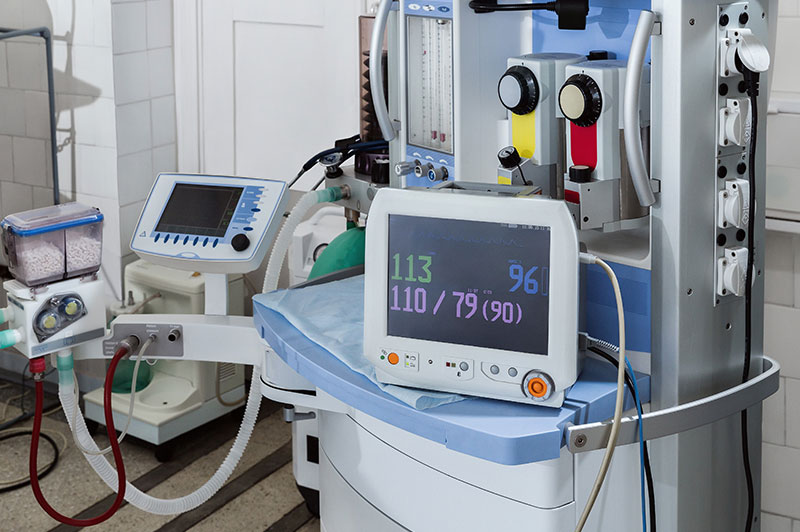
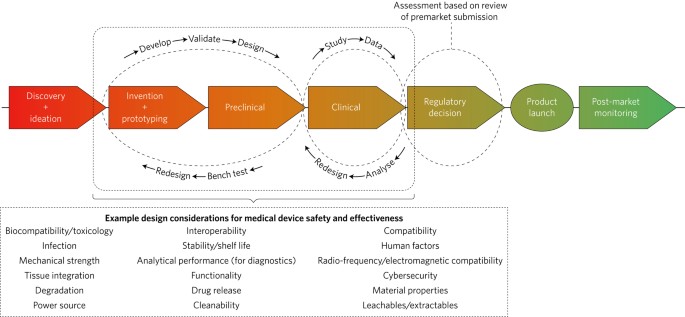
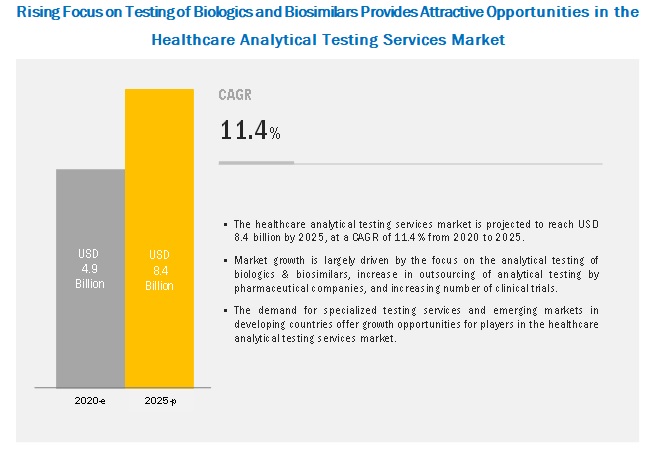
When establishing shelf life claims it must be recognized that the data obtained from accelerated aging testing is based on conditions intended to simulate the effects of aging on and between the materials involved.
Accelerated shelf life testing medical device.
Medical device shelf life aging studies.
Shelf life accelerated aging every medical device is required to be labeled with an expiration date that is supported by shelf life data.
The shelf life of a product may vary between different countries regions depending on regulatory requirements.
The shelf life for a combination product is determined from drug stability device aging and sterile barrier aging with the shortest estimate determining the overall shelf life.
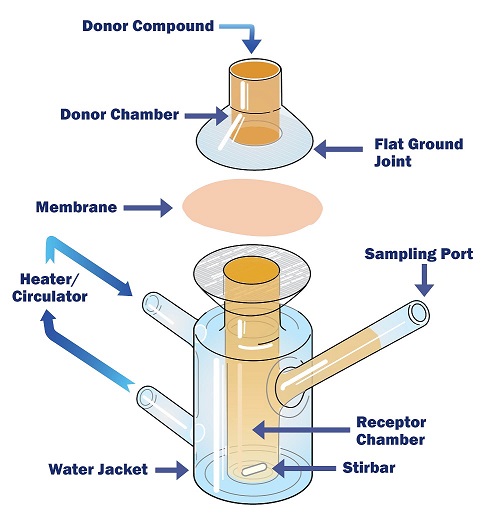
Both accelerated and real time aging should be done on packages that have undergone worst case sterilization.
There are many different endpoints that can be used to assess the shelf life of a medical device including sterility or package integrity so it is important that endpoints and test methodology are decided upon before testing is begun.
These accelerated tests help pinpoint possible seal and burst strength faults leaks and film delamination in medical device and pharmaceutical packaging.
It is used to simulate real shelf life aging and is conducted to validate shelf life claims and document expiration dates.
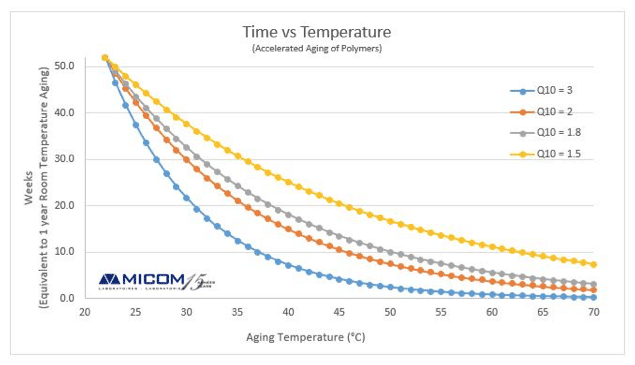
Accelerated aging temperature c typical values are 50 c.
Shelf life to be simulated days days.
Inform readers of the food and drug administration fda regulations and policies relating to shelf life of medical devices.
Accelerated aging time calculator astm f1980 07.
Long term accelerated shelf life testing ich due to the integration of active pharmaceutical ingredients api and antimicrobial agents more medical devices are considered to be combination products than in years past.
Commonly referenced medical device standards and fda guidance documents.
Accelerated aging tests are employed to generate this data for design history files technical dossiers and 510 k submissions while concurrently running real time studies.
Medical device manufacturers wishing to gather data on the shelf life of their products may subject their devices to long term stability studies or accelerated aging studies.
Time typically undergo both accelerated and real time testing to establish the shelf life of the seal.
Accelerated aging data is recognized by regulatory bodies as a conservative estimate of the shelf life but is only accepted until those tests can be repeated on real time aged samples.
Ddl conducts both accelerated aging testing and real time aging testing to help establish shelf life and expiration dates for medical devices packaging and products.
A product can be released to market based upon successful accelerated aging test results that simulates the period claimed for product expiration date 1 year 2 years etc.
Accelerated aging studies can be used for shelf life determination but must later be verified using data from the long term storage conditions.
Accelerated testing is allowed for market launch but must be followed up by real time data.